L. J. Grauke (Research Horticulturist)
USDA-ARS Pecan Breeding & Genetics 10200 FM 50 Somerville, TX 77879 phone 979-272-1402 FAX 979-272-1401 ljg@tamu.eduIn order to monitor flowering in pecan, it helps to understand the flowering system of the tree. This requires the use of specialized words, which will be defined below.
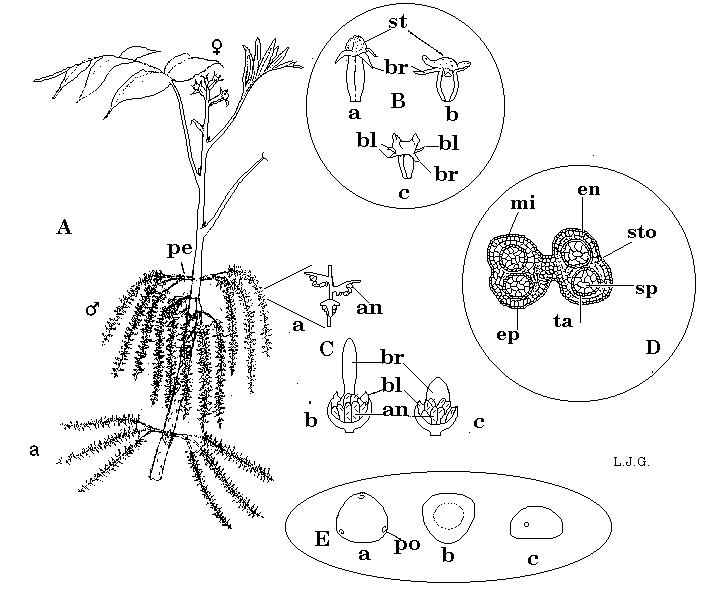
Mature pecan trees bear male and female flowers at different locations on the same tree. Pecan flowers develop from the compound buds, which are composed of two lateral floral or catkin buds and a central mixed bud . As growth resumes in spring, the central mixed bud elongates to form the vegetative shoot, which may terminate in the female (pistillate) inflorescence. The two lateral floral buds each produce a three stalked catkin group, the male (staminate) inflorescence.
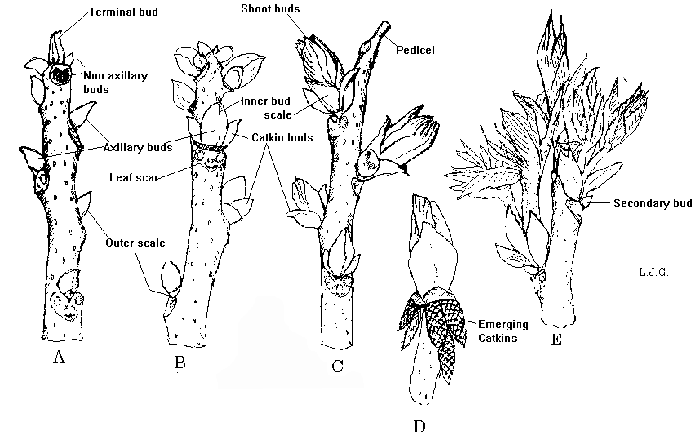
Figure 1. Bud growth of pecan, showing development of lateral floral (staminate) buds.

Figure 2. Flowering in pecan. A: shoot with terminal pistillate (female) spike at end of current season's growth; staminate (male) flowers borne in pairs of 3 stalked catkin groups at base of current season's growth, or (a) from lateral buds in which the vegetative shoot aborts; pe, peduncle; B:pistillate flowers with variable shaped stigmas (st) subtended by 3 bracteoles (bl) and a bract (br). C:male flowers vary in bract (br) structure from protogynous (b) and protandrous (c) cultivars. D: cross section of an anther; ep, epidermis; en, endothecium; mi, middle layers; ta, tapetum; sp, sporogenous tissue (pollen); sto, position of stomium (where anther splits to release pollen); E. Pollen grains; a, distal view of triporate pollen grain; po, pore; b, proximal view, showing enfolding; c, equatorial view showing suboblate shape.
A pecan tree has dichogamous flowering (dicho='two part'; gamy='sexual union'), since male and female flowers on a tree mature at different times. If male flowers dehisce pollen before pistillate flowers are receptive, the tree is protandrous (protos=first; andro=male) and is classified as type I.
Protandrous cultivar 'Clark' (CSV 11-10) shedding pollen on April 28, 2003.
Protandrous cultivar 'Clark' (CSV 11-10) with stigmas not yet receptive on April 28, 2003.
If female flowers are receptive before pollen is shed from catkins, the tree is protogynous (protos=first; gyne=female), and is classified as type II.
Protogynous cultivar 'Choctaw' (CSHQ 2-3) with catkins prior to shedding on April 24, 2003.
Since different trees have different bloom patterns or dichogamies, the species as a whole is termed heterodichogamous (hetero=different; dichogamy=two part bloom). This type of flowering encourages genetic diversity by maximizing outcrossing (Thompson & Romberg, 1985). Patterns of dichogamy are due to a single gene with simple dominance, designated as the P gene. Protogyny is the dominant character, and only one homozygous dominant (PP) cultivar is known: 'Mahan'. All 'Mahan' progeny are protogynous (either PP or Pp), since they must receive the dominant gene from 'Mahan' . Protandrous cultivars have homozygous recessive genes (pp). With normal outcrossing in native populations, about half of the trees will be protogynous and half protandrous (Thompson & Romberg, 1985) .
The separation of male and female bloom periods for an individual tree may be complete, or the timing of pollen shed may overlap stigma receptivity (incomplete dichogamy). When a tree has complete separation of male and female bloom, it must be cross pollinated by another tree. Without pollination, nutlets dehisce shortly after bloom, and no crop is set. If the tree has incomplete dichogamy, it may be partially self-pollinated, allowing for some nut set. Self pollination is undesirable, however, since it has been shown to reduce nut quality (Romberg and Smith, 1946; Marquard, 1988). Pecan cultivars have been evaluated to determine their bloom pattern, and the timing of their pollen shed and pistil receptivity in relation to other cultivars. Although cultivars are consistent in the pattern of flower maturation (i.e. protogynous cultivars consistently mature female flowers first, while protandrous cultivars consistently mature male flowers first), there are seasonal differences in the timing of flower development and in the amount of bloom overlap (Grauke and Thompson, 1996). A cultivar may show total separation of pollen shed and pistil receptivity in some years while the same cultivar may have significant overlap of pollen shed and pistil receptivity the following year . When planning an orchard, growers should study records of cultivar bloom patterns (collected locally, if possible) over several years (see Worley et al.,1992).
Staminate inflorescence (Male flowers)
Differentiation
The staminate inflorescence (catkin group) is initiated during the spring prior to pollen shed. Two catkin groups are differentiated on opposite sides of the shoot bud, and are enclosed in separate inner scales. Sometimes, another pair of catkin groups are formed inside the inner scales of the central bud, opposite to each other and at right angles to the first two catkin groups. Each catkin group is enclosed by its own inner bud scales, and the entire compound bud, including lateral catkin groups and the shoot bud, is enclosed by the outer bud scale.
Catkin development progresses to different stages in protandrous and protogynous cultivars: protandrous cultivars initiate anthers on catkins in the buds of the staminate inflorescence during the summer prior to pollen shed; protogynous cultivars initiate anthers on catkins during the spring that pollen is shed (Wetzstein and Sparks, 1984; Haulik and Holtzhausen, 1988). Luza and Polito (1988) found that when walnut trees resumed growth in the spring, staminate flower differentiation resumed in protandrous clones prior to resumption of differentiation in protogynous clones. Comparable studies have not been performed for pecan.
Structure and development
The staminate inflorescence, or catkin groups, are composed of three aments or catkins, joined to a common stalk, or peduncle.
Protogynous cultivars typically have long, thin catkins, while protandrous cultivars typically have catkins which are shorter and of greater diameter (Woodroof and Woodroof, 1929). Regardless of dichogamy class, the central catkin of a catkin group is usually the longest.
Cultivars vary in the quantity of catkins produced. Some cultivars, such as 'Desirable'and 'Cape Fear', are known as heavy catkin producers, while others typically produce fewer catkins. Catkin production for a cultivar should be assessed at the beginning of anther dehiscence for that cultivar to insure maximum catkin presence. Assessments made over entire orchards on a single date may be inaccurate due to variation in catkin emergence between cultivars.
Each catkin is composed of many individual staminate flowers: ~72/catkin in protandrous cultivars; ~123/catkin in protogynous cultivars (from data in Woodroof, 1924). Each individual staminate flower is composed of a central bract and two lateral bracteoles. Protogynous cultivars typically have male flowers with long, thin bracts, while protandrous cultivars typically have male flowers with short, broad bracts (Woodroof, 1924)(Fig. 2).
From three to seven stamen develop in each staminate flower. The stamen is composed of the anther, where the pollen develops, and the filament, or stalk, which attaches it to the flower. In pecan, the filament is so short that it is inconspicuous. When mature, each anther will have four pollen sacs, or thecae (Fig. 2).
Pollen is developed in the pollen sacs. Woodroof (1930) calculated that each pollen sac contained an average of 365 pollen grains. Using estimates provided by Woodroof (1924, 1930) pollen production at various floral organizational levels can be estimated (Table 1). Pollen grains are free within the pollen sac 15 to 20 days before pollen is shed (Woodroof, 1930). Shortly before dehiscence, the four pollen sacs fuse to form two chambers by the dissolution of the separating wall. The anther wall is two cell layers thick, with an outer epidermis and an inner endothecium. Pollen is shed when the pollen sac splits open along a longitudinal slit (stomium). The opening of the anther is caused by drying and contraction of the outer layer in relation to the inner layer. When moistened, the anther has the ability to reclose (Woodroof, 1930).
Anther dehiscence is hastened by increasing temperature under dry conditions, but does not occur under moist conditions, regardless of temperature (Table 2, from data in Woodroof, 1930). Woodroof and Woodroof (1929) reported that pecan pollen continued maturation but did not dehisce if relative humidity exceeded 85%, with subsequent dry conditions resulting in periods of very heavy shed. Sustained high winds coupled with low humidity tend to shorten the period of effective pollination, both by speeding pollen dehiscence and by reducing the period of pistillate receptivity (Woodroof and Woofroof, 1929). Conversely, high humidity delays pollen dehiscence and extends the period of pistil receptivity. The time required for dehiscence under optimum conditions is summarized in Table 3.
Cultivars may vary in the duration of pollen shed, with some cultivars, such as 'Caddo' having the reputation for a short period of shed, while cultivars such as 'Wichita' usually shed pollen longer. The duration of pollen dehiscence for a cultivar may vary greatly in different seasons, as a function of variable weather, and has the potential for extreme variability in different locations across the range of climates where pecan is grown.
Pistillate inflorescence
Differentiation
Female flowers are differentiated during early stages of bud growth in the spring. Wetzstein and Sparks (1983) found that flowers were differentiated at bud swell, after outer scales were split, but prior to inner scale split. There were no apparent differences in time of differentiation of pistillate flowers by protogynous and protandrous cultivars. In walnut, pistillate flowers are differentiated in the season prior to bloom, and proceed to different stages in protogynous and protandrous individuals (Polito and Li, 1985).
Although all compound buds on the previous seasons shoot could potentially form new shoots in the spring, strong apical dominance in pecan usually limits growth (and therefore fruiting) to only two or three compound buds near the terminal portion of the shoot. Lateral buds in basal positions often initiate growth, but abort the shoot tip, forming only catkin groups. When terminal shoots are damaged by early spring freezes, secondary or tertiary buds may break which still have the potential to differentiate flowers.
Structure and development
Pistillate flowers are borne in a spike at the end of the current season's shoot. The basal flowers are the oldest, while the very youngest flowers at the apex are often underdeveloped and abort in the first drop. The number of flowers produced on a single inflorescence varies with shoot length, cultivar, and season.
Pistillate flowers consist of a bilobed stigma on a stigmatic disk surrounded by 3 bracteoles and a bract. The bracteoles and bract are fused at the base to form the involucre or shuck (Manning, 1940)(Fig. 2).
As pistillate flowers mature, the stigmatic surface becomes more prominent and may become red in some cultivars. Receptivity has been judged by the presence of a "viscous fluid" on the stigmatic surface (Adriance, 1931; Mullenax, 1970; Woodroof and Woodroof, 1926), by adherence of applied pollen to the stigmatic surface (Smith and Romberg, 1941; Madden and Brown, 1973), and by calculations based on stigma drying (Dodge, 1939; Stuckey, 1916). The color of stigmas is a trait which cannot be accurately relied upon as an index, since color varies between cultivars from deep red (as in 'Success' and 'Pawnee') to vivid green (as in 'Stuart'). Variation in shape and size of pistillate flowers and stigmatic surfaces at the time of receptivity further complicates the problem (Fig 2). In general, pistillate flowers of protogynous cultivars become receptive at smaller size than those of protandrous cultivars. Within the Pecan Breeding Program, adherence of pollen to the stigma is considered the only reliable method of evaluating stigma receptivity. Dead pollen is dusted on the stigma. If pollen is retained after blowing (evaluated with hand lens if necessary), it is receptive.
In the absence of pollination (as in damp, cloudy, weather, or when pistillate flowers are protected in casings) stigmatic surfaces may remain receptive for a week. However, in dry, windy conditions, stigmatic surfaces may be quickly desiccated, with effective periods of receptivity greatly reduced. If the stigma receives pollen, the stigmatic cells collapse and dry after pollen hydration and germination (Wetzstein and Sparks, 1989), causing the stigma to appear brown and dried.
Literature Cited
Adriance, G.W. 1931. Factors influencing fruit setting in the pecan. Bot. Gaz. 91:144-166.
Dodge, F.N. 1939. Some blossoming relationships found in a study of the dichogamy of pecan varieties. Proc. Amer. Soc. Hort. Sci. 37:503-508.
Grauke, L. J. and Tommy E. Thompson. 1996. Variability in pecan flowering. Fruit Varieties Journal 50:140-150
Haulik, T. K. and L. C. Holtzhausen. 1988. Anatomy of staminate flower ontogeny of the pecan as determined by scanning electron microscopy. S. Afr. J. Plant Soil 5(4):205-208.
Luza and Polito. 1988. Microsporogenesis and anther differentiation in Juglans regia L. A developmental basis for heterodichogamy in walnut. Bot. Gaz. 149:30-36.
Madden, G. D. and E. J. Brown. 1973. Blossom dates of selected pecans. Pecan Quarterly 7(1):17-19.
Madden, G. D. and E. J. Brown. 1975. Here are methods to improve pollination. Pecan Quarterly 9(4):10-12.
Manning, W. E. 1940. The morphology of the flowers of the Juglandaceae. II. The pistillate flowers and fruit. Amer. J. Bot. 27:839-852.
Marquard, R. D. 1988. Outcrossing rates in pecan and the potential for increased yields. J. Amer. Soc. Hort. Sci. 113:84-88.
Mullenax, R. H. 1970. Bud ontogeny, flowering habits, and disease resistance studies of pecan, Carya illinoensis (Wang.) K. Koch. PhD. dissertation, Louisiana State Univ., Baton Rouge, LA.
Nakayama, R. M. 1967. Pecan variety characteristics. New Mexico Agr. Expt. Sta. Bull. 520.
Polito, V. S. and N. Y. Li. 1985. Pistillate flower differentiation in English walnut (Juglans regia L.): developmental basis for heterodichogamy. Sci. Hort. 26:333-338.
Romberg, L.D. and C.L. Smith. 1946. Effects of cross-pollination, self-pollination, and sib-pollination on the dropping, the volume, and the kernel development of pecan nuts and on the vigor of the seedlings. Proc. Amer. Soc. Hort. Sci. 47:130-138.
Smith, C. L. and L. D. Romberg. 1941. Pollen adherence as a criterion of the beginning of stigma receptivity in the pecan. Proc. Texas Pecan Growers Assoc. 21:38-45.
Sparks, D. 1992. Pecan Cultivars: The orchards foundation. Pecan Production Innovations, Watkinsville, GA.
Stuckey, H. P. 1916. The two groups of varieties of the Hicoria pecan and their relation to self-sterility. Ga. Exp. Sta. Bull No. 124.
Thompson, T. E. and L. D. Romberg. 1985. Inheritance of heterodichogamy in pecan. J. Heredity 76:456-458.
Thompson, T.E., F. Young. 1985. Pecan Cultivars-- Past and Present. Texas Pecan Growers Assoc. Inc. 265pp.
Wetzstein, H. Y. and D. Sparks. 1983. Morphology of pistillate flower differentiation in pecan. J. Amer. Soc. Hort. Sci. 108:997-1003.
Wetzstein, H. Y. and D. Sparks. 1984. The morphology of staminate flower differentiation in pecan. J. Amer. Soc. Hort. Sci. 109:245-252.
Wetzstein, H. Y. and D. Sparks. 1989. Stigma-Pollen interactions in pecan. J. Amer. Soc. Hort. Sci. 114:355-359.
Woodroof, J. G. 1924. The development of pecan buds and the quantitative production of pollen. Georgia Experiment Station Bulletin 144.
Woodroof, J. G. 1930. Studies of the staminate inflorescence and pollen of Hicoria pecan. J. Agri. Res. 40:1059-1104.
Woodroof, J. G. and N. C. Woodroof. 1926. Fruit-bud differentiation and subsequent development of the flowers in the Hicoria pecan. J.Agr. Res. 33:677-685.
Woodroof, J. G., and N. C. Woodroof. 1929. Flowering and fruiting habit of the pecan. Proc. National Pecan Assoc. 28:128-136.
Worley, R. E., S. K. Dove, B. G. Mullinix, Jr. and M. Smith. 1992. Long-term dichogamy of 80 pecan cultivars. Scientia Horticulturae 49:93-101.